

If Calcium has the number 20, it has 20 protons (and electrons). 6 of them are naturally occurring, 5 of which are stable.Ĭalcium is used by many forms of life to make shells and bones. Electrons have no relevant mass, and protons and neutrons both have the mass of 1u. Calcium is an essential element for living organisms.
Where should the student look number of neutrons. 4.2- Isotopes of the same element have the same. It is used a reducing agent in the extraction of thorium, zirconium and uranium. While looking at calcium (Ca) on the periodic table, a student needs to find an element with a greater atomic mass in the same period. Justify the following statements: 4.1- The nucleus of the stable isotope of fluorine contains 10 neutrons. They each have the same number of protons. Since a stable atom has a net charge of 0.

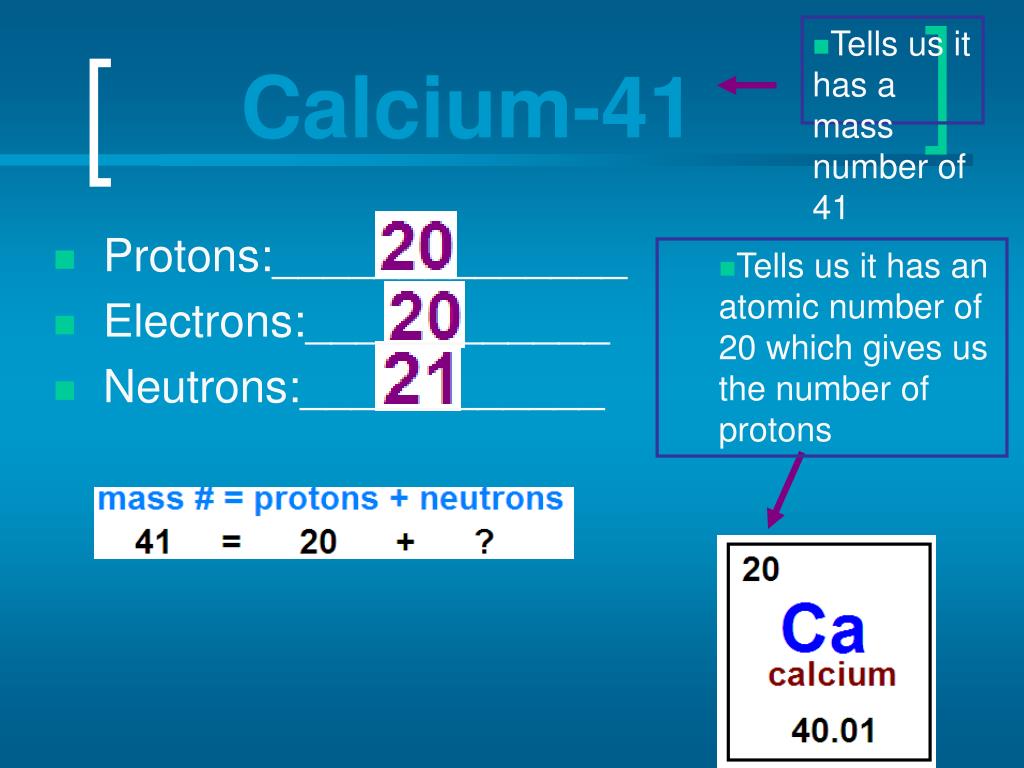
According to the list of elements given inside the. Calcium is the 20th element, with 20 protons (since the number of protons directly changes the element itself).
#Ca element neutrons plus
They each have the same number of protons. (a) The superscript 197 is the mass number, the sum of the number of protons plus the number of neutrons. They each have the same number of neutrons. » Boiling Point » Melting Point » Abundant » State at STP » Discovery Year. Melting point of Calcium is 839 ☌ and its the boiling point is 1487 ☌. In our example, this is: 14 (atomic mass) 6 (number of protons) 8 (number of neutrons). Atomic weight of Calcium is 40.078 u or g/mol. the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. Molecules consist of the same element with different numbers of atoms and chemical structure are called a) Ions b) Neutrons c) Allotropes d) Isotopes. They each have the same number of neutral particles. A mass number number of protons and neutrons in the most common (or most stable) nucleus. Since the vast majority of an atom’s mass is found its protons and neutrons, subtracting the number of protons (i.e. The periodic table has a special shape that will become important to us when we consider the organization of electrons in atoms (Chapter 8).Calcium is a soft grey metallic element belonging to group 2 of the periodic table. Elements - Quiz (80) 3.4 (8 reviews) What is true about all uranium atoms They each have the same number of nuclear particles.

The elements on the periodic table are listed in order of ascending atomic number. \), while one may view a more extensive periodic table from another source.


 0 kommentar(er)
0 kommentar(er)
